With developing and implementing laboratory and environmental monitoring programs, determining the appropriate balance between an efficient allocation of resources and a sufficient frequency of monitoring data in order to derive meaningful conclusions is very important.
Best Practices for Lab Monitoring - Before, During, After Implementation
The key purpose of a lab equipment and facility monitoring program is to provide critical information on the quality of equipment and facility conditions. Identifying best practices and solutions that help organizations achieve this and alleviate the burden of monitoring the many areas of life science facilities can often be one of the more difficult challenges that organizations face.
When a lab monitoring solution is identified, and best practices and standard operating procedures (SOPs) are clearly defined, laboratory and environmental monitoring ensure equipment and facility conditions are functioning optimally to perform the complex scientific processes life science organizations need to operate on a daily basis in order to remain innovative in this fast-paced industry.
The FDA establishes clear details in guidance documents indicating that one of the most important laboratory controls is an environmental monitoring program. Environmental monitoring data provides the necessary quality information on drug research, storage, and manufacturing environments. The benefits of best practices for laboratory and environmental monitoring can be seen across all areas of life science product development.
Alert and Action Levels for Equipment and Facility Conditions
In determining the proper parameters for an equipment and facility monitoring system, there needs to be organizational agreement on the scope and purpose of what is to be monitored. The purpose of a monitoring program is to gain insight through documentation and reporting for the state of control of equipment and facility conditions, which can help determine the quality of products from start to finish.
Deciding the appropriate procedures for alerts and notifications in the event of deviations is a very important step in defining best practices for lab monitoring. When organizations rely on manual records and outdated procedures for receiving notifications during any deviations of lab equipment and facility conditions outside of preset parameters, the risks of loss and catastrophic failures increase exponentially. Utilizing robust lab monitoring solutions help to alleviate these issues.
A real-time lab monitoring solution allows more in-depth understanding for the quality of samples, reagents, and other important research materials stored in lab equipment and environments that can be affected by air, personnel, water, particles, and many other conditions. A viable monitoring solution also ensures compliance with various strict regulatory specifications from myriad organizations like the Food and Drug Administration (FDA), International Organization for Standardization (ISO), United States Pharmacopeia (USP), and many others.
XiltriX 24/7, Real-Time Lab Monitoring
XiltriX provides state-of-the-art, real-time laboratory monitoring services. The XiltriX SafetyNet team of experts implements industrial-grade hardware and cloud-based software to monitor laboratory equipment functionality and facility conditions. XiltriX provides managed monitoring services and is there every step of the way to set up and configure the system which includes configuration of alarm limits, user roles, automated reports, and much more.
The XiltriX system sends immediate notifications and alerts with physical alarms, text messages, emails, and/or phone calls to the appropriate personnel if any conditions deviate from preset parameters. The XiltriX team also proactively monitors and manages the system 24/7 to alleviate the allocation of internal employees managing the system, make sure everything is functioning optimally, and address every alarm appropriately. In addition, the XiltriX SafetyNet team of experts helps generate automated quality reports, providing value-added insights through data analysis. XiltriX Monitoring-as-a-Service is unlike any other laboratory or environmental monitoring solution on the market.
XiltriX Best Practices for Lab Monitoring--Provided Before, During, and After Implementation
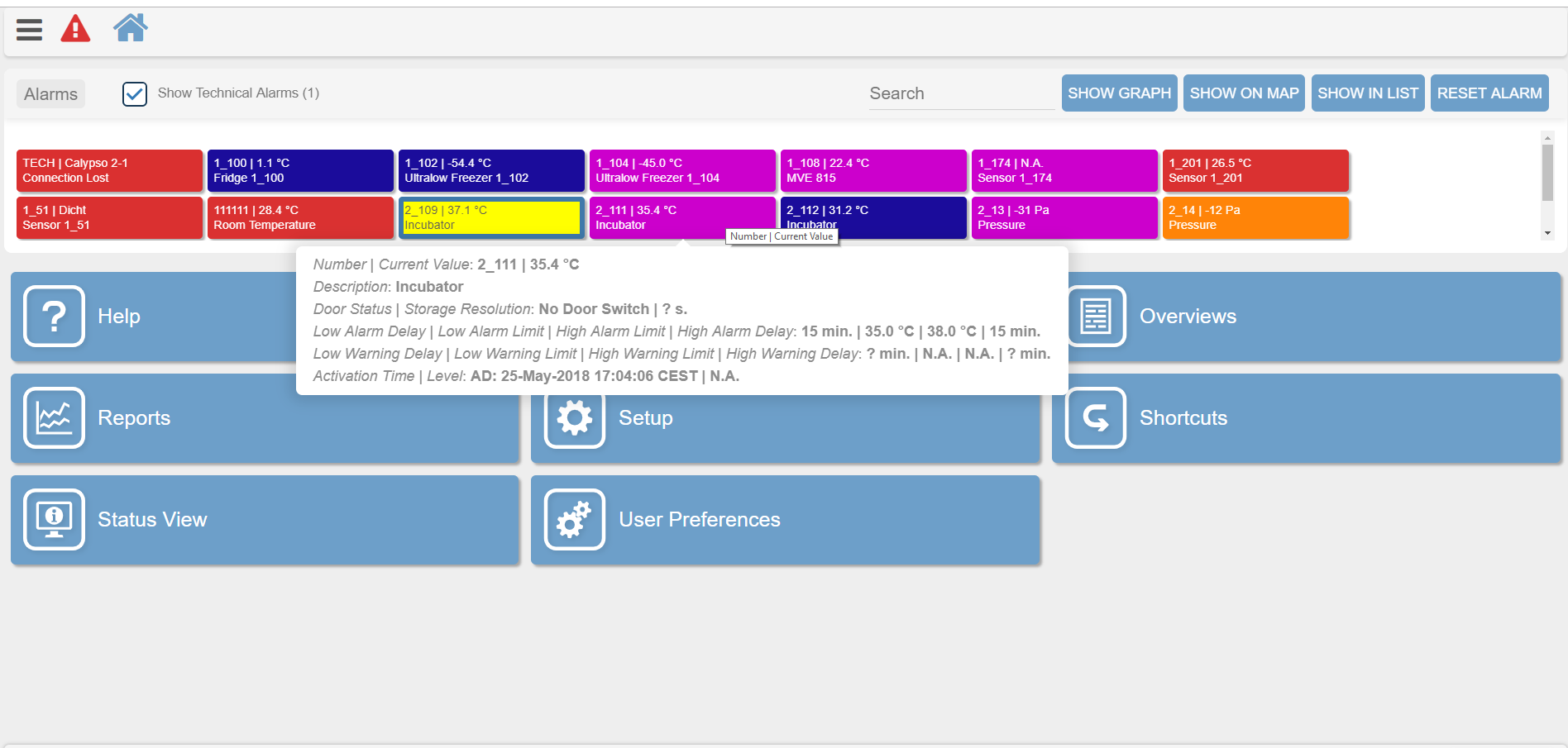
Determine high alarm and low alarm limits for equipment and facility conditions
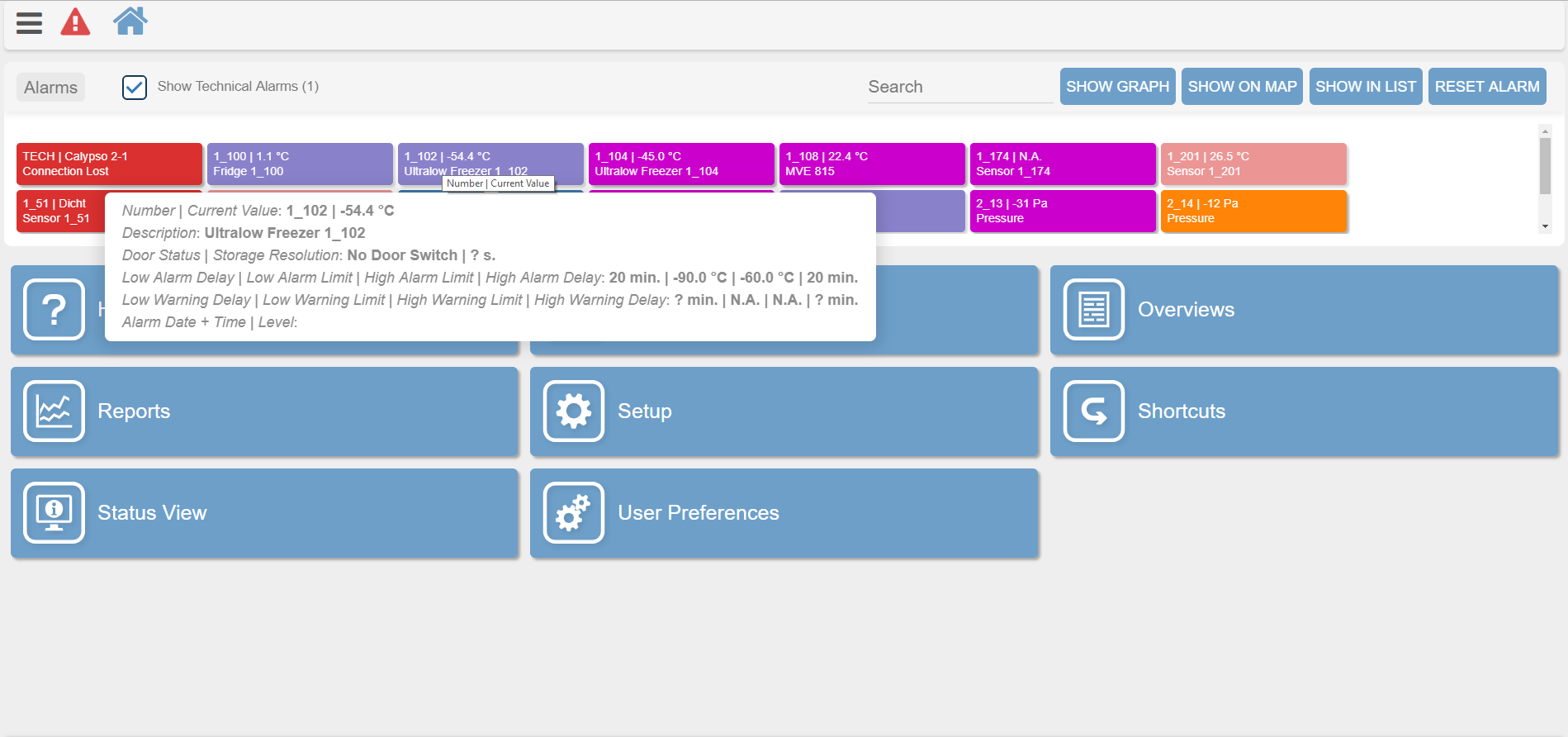
An example of the XiltriX Saturn software showing real-time alarm limit parameters and equipment condition details for an Ultralow Freezer (above) and Incubator (below).
Configure user roles and notification procedures
The XiltriX system allows for a wide variety of configurations and customizations. The XiltriX SafetyNet team helps with all aspects of system setup.
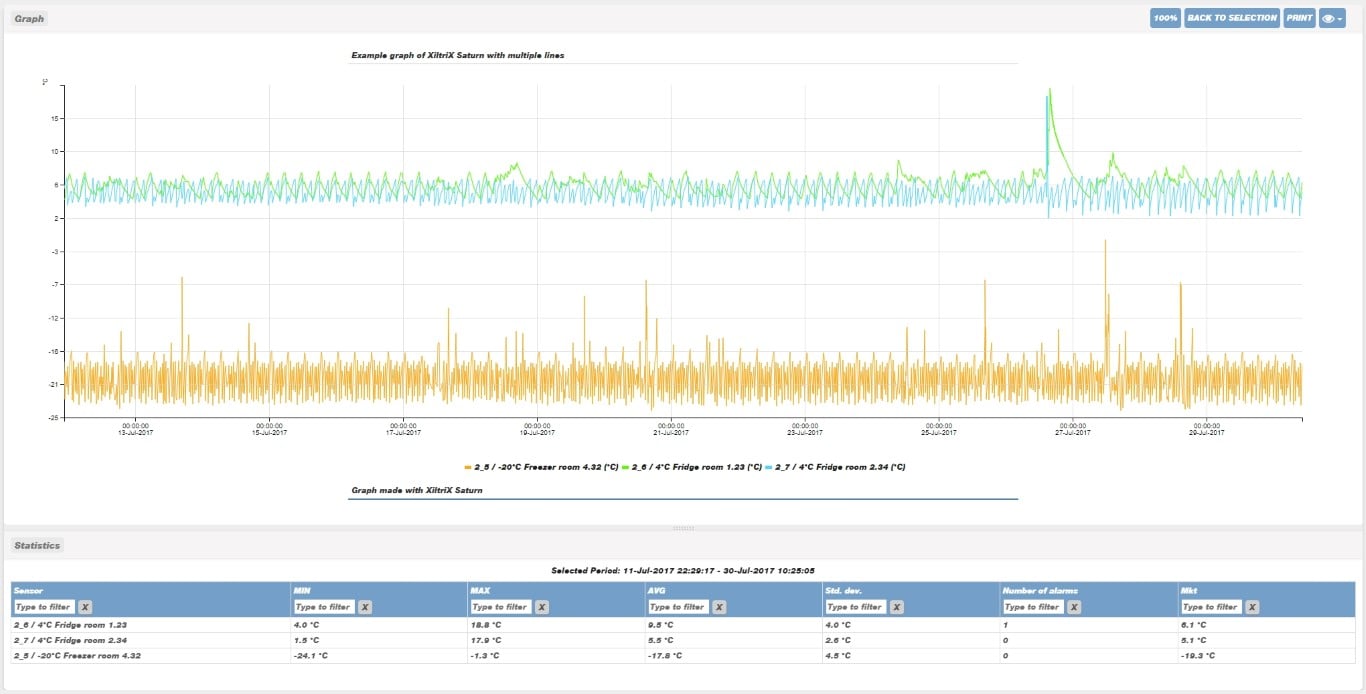
Provide value-added insight with expert data analysis
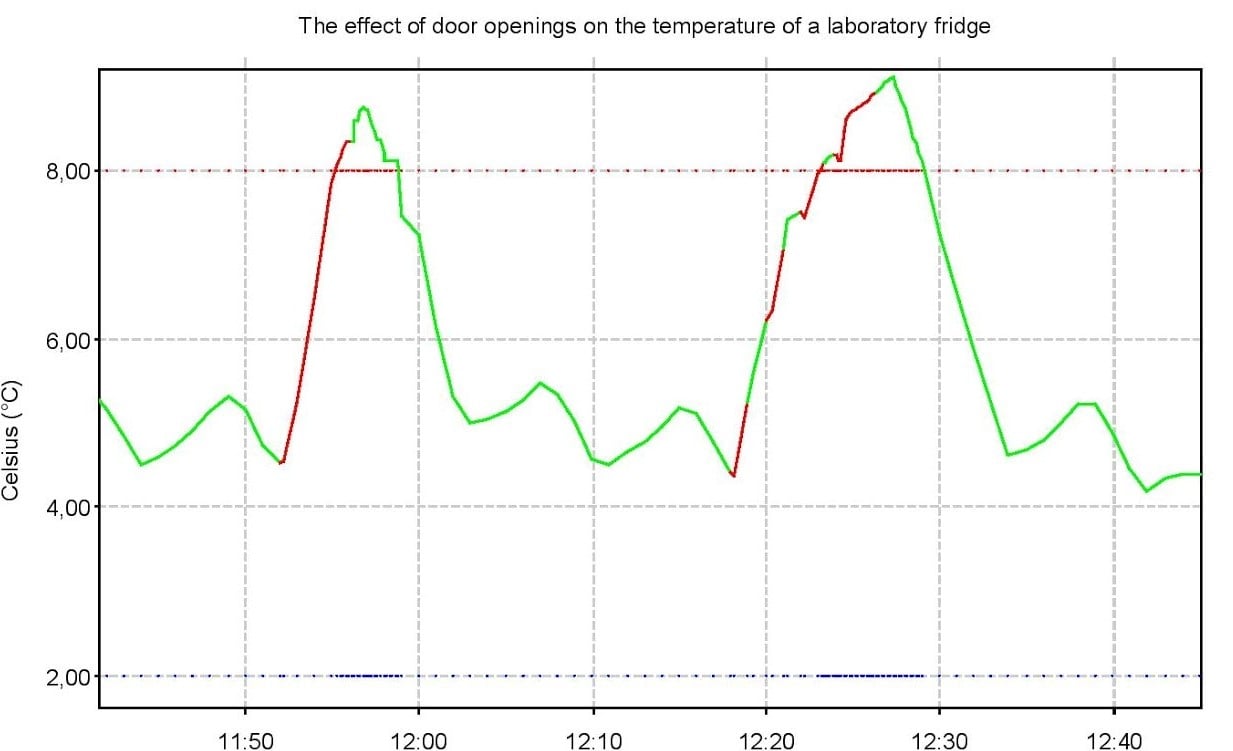
This graph details the prolonged, or frequent, door openings (in red) of a lab refrigerator which caused deviations outside of the high alarm limit. The XiltriX SafetyNet team recommends improving SOPs to alleviate any potential equipment issues or sample damage.
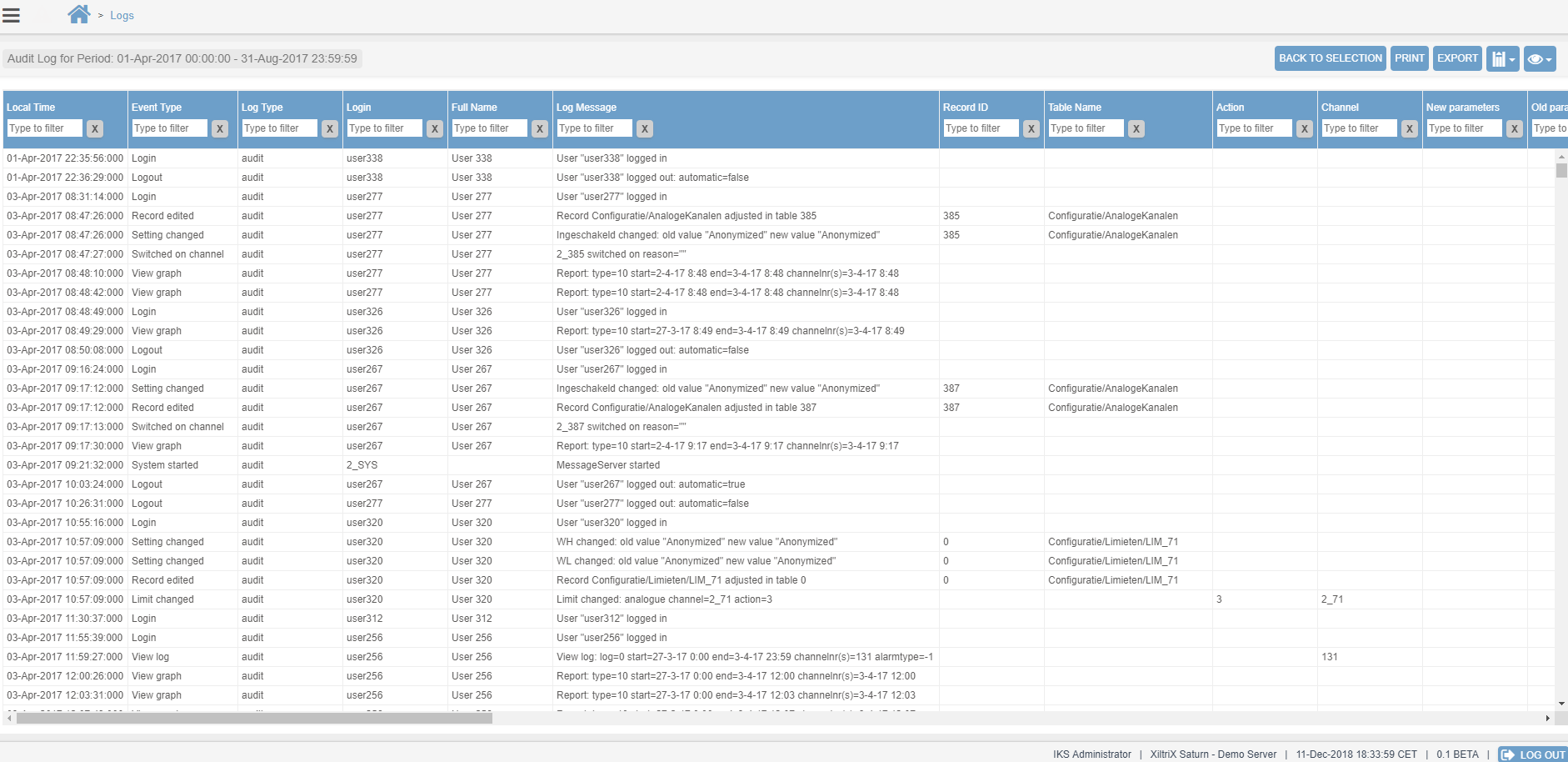
Generate automated reports for quality & compliance
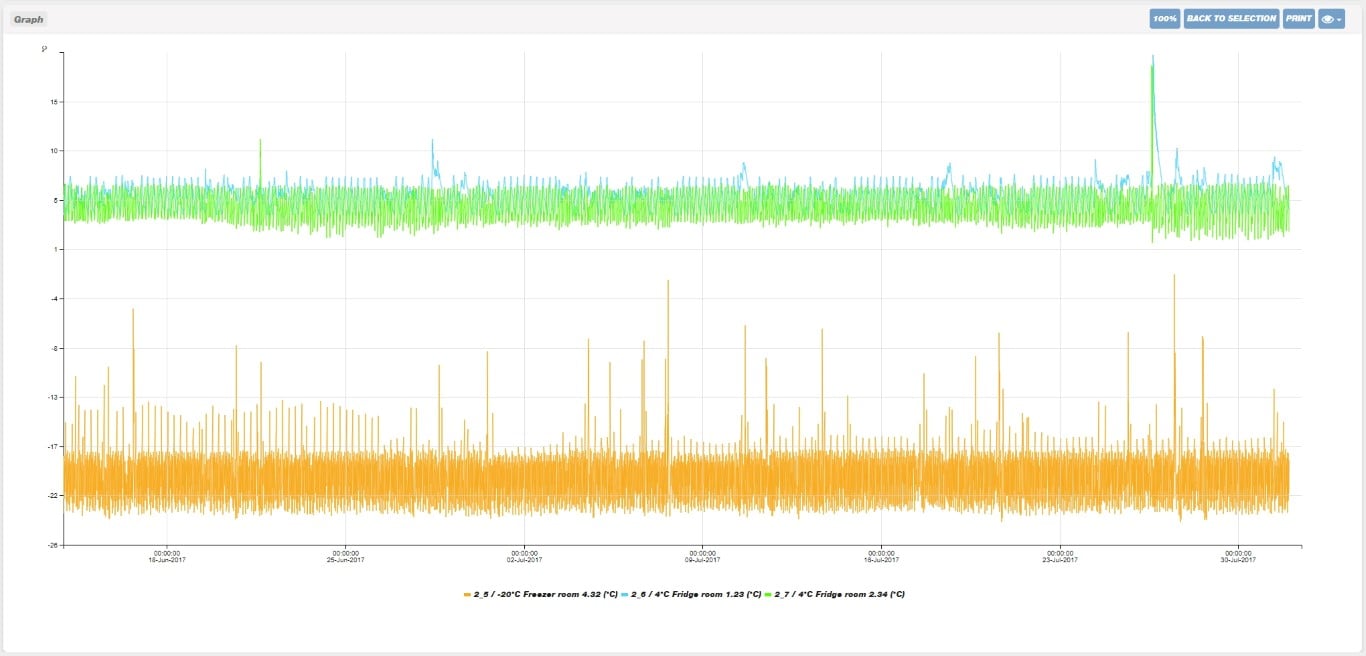
The XiltriX system can generate a variety of reports and graphs for internal & external quality & compliance. From User Audit Reports (Top), to Graphical + Numerical Equipment Reports (Middle), to Graphical Equipment Overlays (Bottom). These reports can be customized to departmental needs and are easily saved or printed for quality & compliance documentation.
Concerned about the security of your facility, projects, and intellectual property, and the purity of your environment? Here's a white paper listing five critical questions to ask when implementing a lab monitoring solution: